Please note that the package insert for the Pfizer mRNA vax has been updated - and is copied below. Please click here to jump to it
The two separate package inserts referenced and discussed below (i.e. the one for recipient and the one for the healthcare professional) can be downloaded (or viewed direct) from this server folder here.
The package inserts for the Pfizer BioNTech COVID-19 vaccine have been split into two separate documents (for recipient and for 'healthcare professional') with one extremely key piece of information being omitted (presumably not accidentally) from the recipient version. The info missing from the recipient's version which I am referring to is as follows (copied from the healthcare professional version):
4.6 Fertility, pregnancy and lactation
...
Fertility
It is unknown whether COVID-19 mRNA Vaccine BNT162b2 has an impact on fertility.
This would seem a particularly important matter given what Dr. Wolfgang Wodarg and Dr. Michael Yeadon highlight in their petition to various medical regulators, as follows (for the full text of Wodarg and Yeadon's submission please see this post here):
XI. Several vaccine candidates are expected to induce the formation of humoral antibodies against spike proteins of SARS-CoV-2. Syncytin-1 (see Gallaher, B., “Response to nCoV2019 Against Backdrop of Endogenous Retroviruses” - https://virological.org/t/response-to-ncov2019-against-backdrop-of-endogenous-retroviruses/396), which is derived from human endogenous retroviruses (HERV) and is responsible for the development of a placenta in mammals and humans and is therefore an essential prerequisite for a successful pregnancy, is also found in homologous form in the spike proteins of SARS viruses. There is no indication whether antibodies against spike proteins of SARS viruses would also act like anti-Syncytin-1 antibodies. However, if this were to be the case this would then also prevent the formation of a placenta which would result in vaccinated women essentially becoming infertile. To my knowledge, Pfizer/BioNTech has yet to release any samples of written materials provided to patients, so it is unclear what, if any, information regarding (potential) fertility-specific risks caused by antibodies is included.
According to section 10.4.2 of the Pfizer/BioNTech trial protocol, a woman of childbearing potential (WOCBP) is eligible to participate if she is not pregnant or breastfeeding, and is using an acceptable contraceptive method as described in the trial protocol during the intervention period (for a minimum of 28 days after the last dose of study intervention).
This means that it could take a relatively long time before a noticeable number of cases of post-vaccination infertility could be observed.
I would be surprised if not informing recipients about such a crucial potential side-effect of the vaccine didn't breach the healthcare professional's legal duties in respect of the information they are required to provide as part of the 'informed consent' process. In fact, purposefully not providing such information (at least in terms of section 4.6 above) would seem to fit the definition of medical malpractice (which in essence is severe medical negligence).
I mean what exactly is going on here? Is it intended that healthcare professionals (presuming they have read the insert intended for them) make a decision for each female of child bearing age - as to whether that female is worthy of being advised about such an important piece of information?
It makes me wonder if this is part of why an army of amateurs are being recruited to give the vaccination and provide the relevant information as part of the informed consent process (i.e. are the 'real' medical professionals keeping themselves at a safe distance?).
As regards legal indemnification for all involved parties, the recently introduced section 345 of The Human Medicines Regulations (2012), states in part (3) of section 345:
(3) None of the following are to be subject to any civil liability for any loss or damage resulting from the use of the product in accordance with the recommendation or requirement—
(a) any holder of an authorisation for the product;
(aa) if there is no holder of an authorisation for the product but the sale or supply of the product is authorised by the licensing authority on a temporary basis under regulation 174, the person responsible for placing the product on the market in the United Kingdom;]
(b) any manufacturer of the product;
(c) any officer, servant, employee or agent of a person within sub-paragraph (a), (aa) or (b);
(d) any health care professional; or
(e) any person, not being a health care professional, who administers the product in accordance with a protocol of the type mentioned in regulation 247A.
So in short, absolutely no one holds legal responsibility for injury whatsoever, that might be caused to an individual by a CV19 vaccine. The only avenue left for compensation for an injured individual will be to attempt to claim a Vaccine Damage Payment from the UK state (max of £120k) about which is written:
Vaccine Damage Payment is a seldom-awarded lump sum for people who have suffered from the adverse effects of a vaccine...
But they will have to meet the requirements laid down by the Government.
They state people must be at least 60 percent disabled as a direct result of receiving one [of the specified vaccines]. (source)
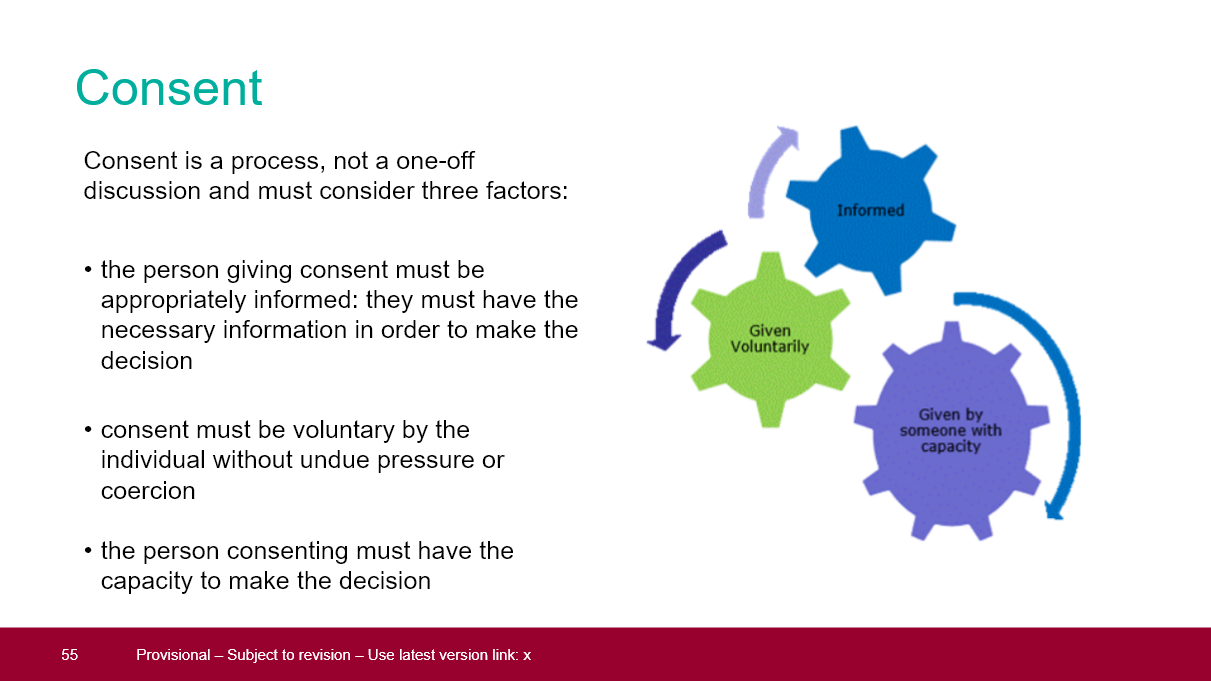
Given the above, it is worth noting that in the UK, by law, no vaccines are compulsory. Consent to receive a vaccine has to be clearly given by the recipient (presuming they are capable of giving consent). In fact the training material for the newly recruited 'army of vaccinators' clearly states that they must abide by this principle. Below are four slides from an official PowerPoint presentation for 'healthcare professionals' training to vaccinate with CV19 vaxes (the full presentation can be viewed/downloaded here).



The updated package insert for the Pfizer/BioNTech COVID-19 vaccine states the following statements:
4.6 Fertility, pregnancy and lactation Pregnancy
There is limited experience with use of the COVID-19 mRNA Vaccine BNT162b2in pregnant women. Animal studies do not indicate direct or indirect harmful effects with respect to pregnancy, embryo/foetal development, parturition or post-natal development (see section 5.3). Administration of the COVID-19 mRNA Vaccine BNT162b2 in pregnancy should only be considered when the potential benefits outweigh any potential risks for the mother and foetus.
...
Fertility : Animal studies do not indicate direct or indirect harmful effects with respect to reproductive toxicity (see section 5.3).
and:
5.3 Preclinical safety data
Non-clinical data reveal no special hazard for humans based on a conventional study of repeat dose toxicity.
Reproductive toxicity: Reproductive and developmental toxicity were investigated in rats in a combined fertility and developmental toxicity study where female rats were intramuscularly administered with the COVID-19 mRNA Vaccine BNT162b2 prior to mating and during gestation (receiving 4 full human doses that generate relatively higher levels in rat due to body weight differences, spanning between pre-mating day 21 and gestational day 20). SARS-CoV-2 neutralizing antibody responses were present in maternal animals from prior to mating to the end of the study on postnatal day 21 as well as in foetuses and offspring. There were no vaccine-related effects on female fertility, pregnancy, or embryo-foetal or offspring development. No data on the COVID-19 mRNA Vaccine BNT162b2 are available on vaccine placental transfer or excretion in milk.
Just to check I have understood the situation correctly... It is aimed to vax almost the entire population of young fertile cis females on the earth, based on a rat study!!!